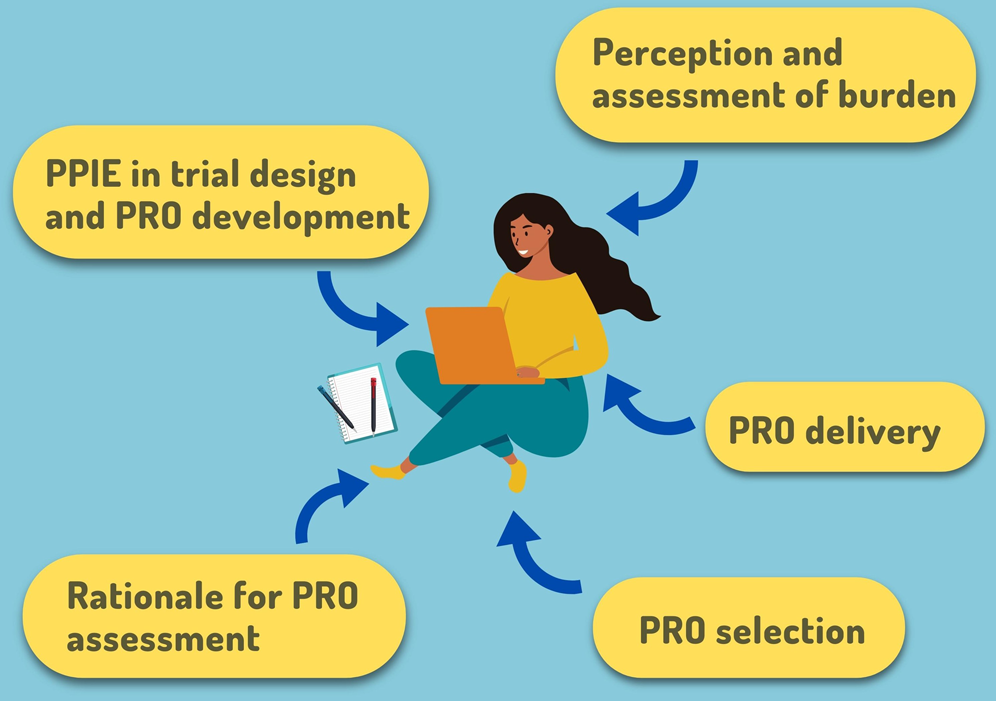
Key considerations to reduce or address respondent burden in patient-reported outcome (PRO) data collection | Nature Communications

Patient-reported outcomes with atezolizumab plus bevacizumab versus sorafenib in patients with unresectable hepatocellular carcinoma (IMbrave150): an open-label, randomised, phase 3 trial - The Lancet Oncology
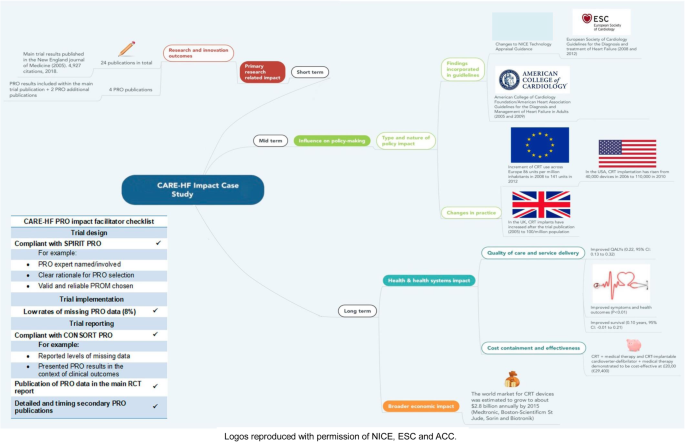
The impact of patient-reported outcome (PRO) data from clinical trials: a systematic review and critical analysis | Health and Quality of Life Outcomes | Full Text
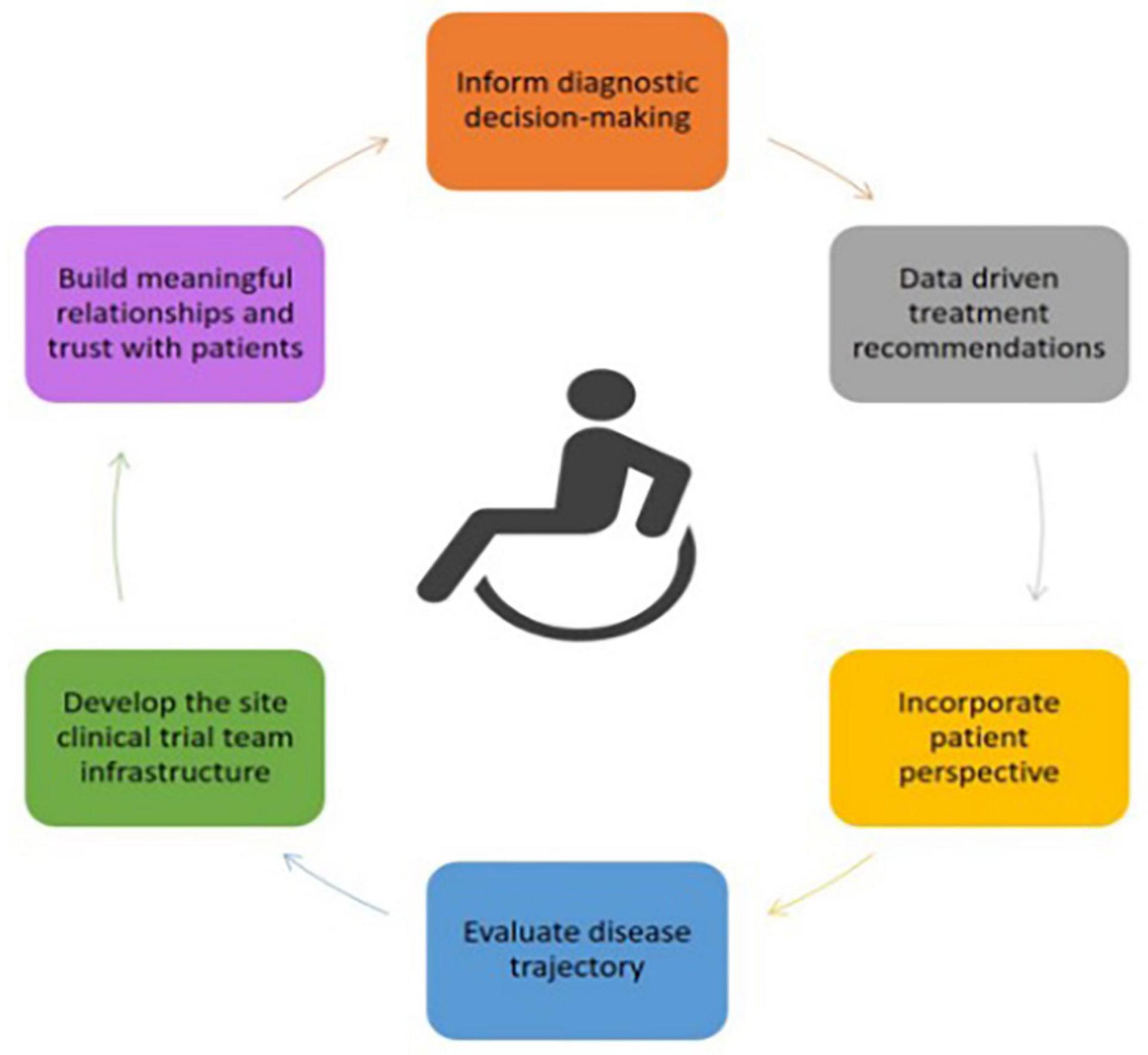
Frontiers | Consensus Guidelines for Improving Quality of Assessment and Training for Neuromuscular Diseases
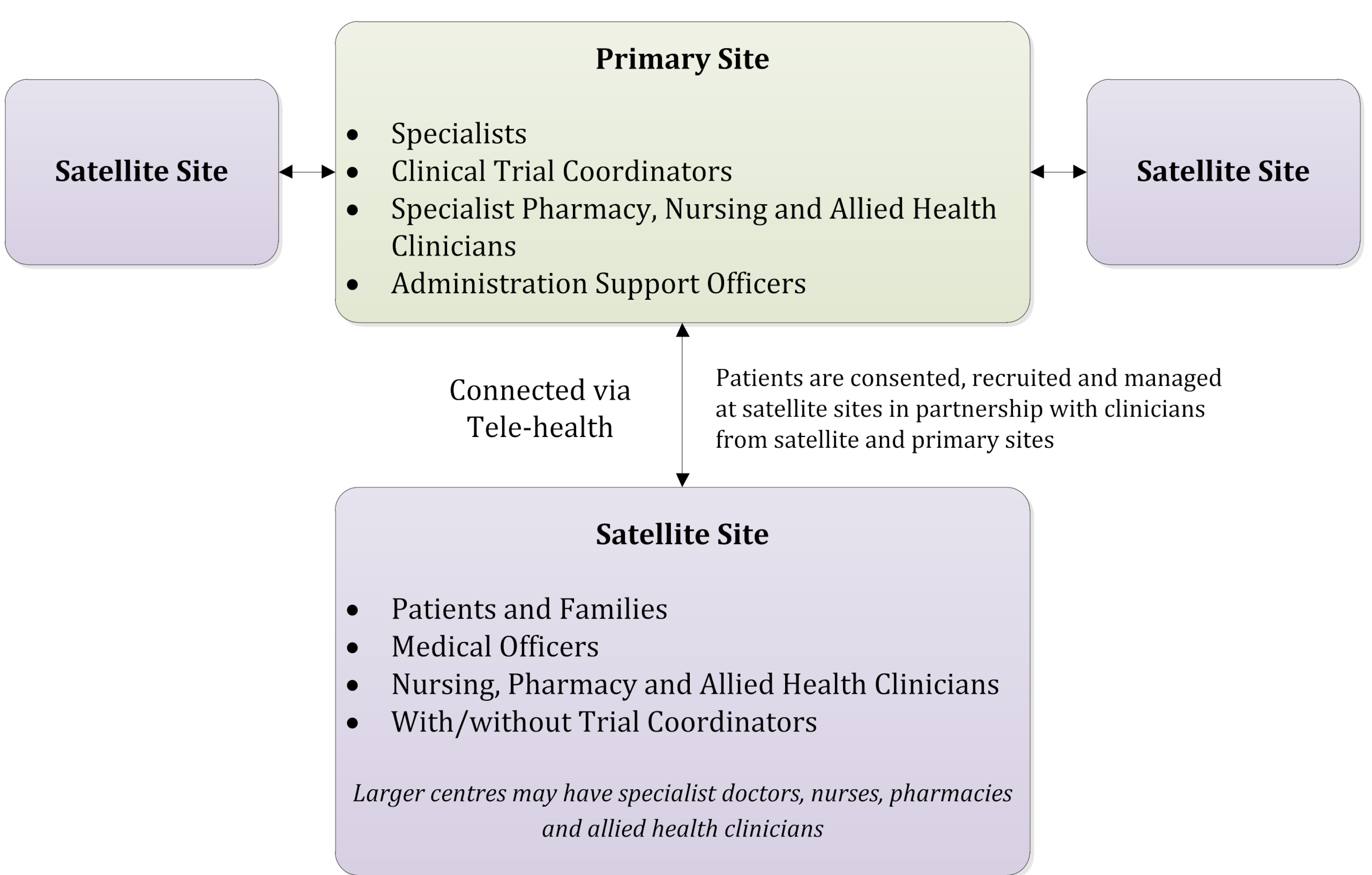
Current Oncology | Free Full-Text | CRAFT—A Proposed Framework for Decentralized Clinical Trials Participation in Canada